机会氮氧化物
In natural gas processing, wellhead gas is usually passed through field separator units to remove hydrocarbon condensate and water. If hydrogen sulfide is present, it is often removed using a sweetening process, which involves absorption in an amine solution before the gas can be utilised. The overhead from the amine regenerator is frequently sent to a thermal oxidiser, (often called an incinerator) in which the H2S and other combustibles are oxidised to form sulfur dioxide, carbon dioxide and water vapour, before they are vented to the atmosphere via an elevated stack. Glycol dehydrators, sulfur recovery units and other processes may also generate tail gases or vent gases that have unacceptable levels of H2S and CO, as well as VOCs that require mitigation. Tank vents sometimes require treatment before the gases can be released to the atmosphere. A thermal oxidiser is often the simplest and most cost effective way to achieve effective destruction of H2S and other combustibles in these
waste gas streams.
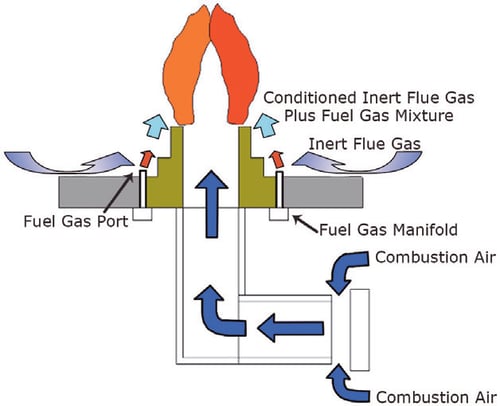
The function of a thermal oxidiser is to provide an environment in which the combustion reaction of the waste gas can be sustained and completed. The combustion chamber temperature is controlled by modulating the firing rate of the burner in order to maintain a temperature that is high enough to ensure completion of the combustion reaction, but not high enough to damage the combustion chamber’s refractory lining. A properly designed thermal oxidiser is sized to provide a residence time in the combustion chamber that usually exceeds one second from the waste gas injection point to the end of the chamber. This allows the thermal oxidiser to incinerate all waste gases before the treated flue gas is dispersed into the atmosphere.

Successful installations
One example of a successful ultra low NOx burner installation is located in a sulfur recovery unit (SRU) tail gas thermal oxidiser operating at a Canadian refinery. The tail gas waste stream contained a significant concentration of ammonia (NH3) that averaged in excess of 400 ppmv. If burned in a high temperature, high oxygen environment, the amount of bound nitrogen could be converted to NOx at a rate of 30% or greater. However, by using a specialised waste gas injection method with a controlled amount of excess air, along with an ultra low NOx burner design, the test results in Table 1 show that the combined thermal NOx plus fuel bound NOx is 18.5 ppmv at 3% oxygen.
挑战
Ultra low NOx burners similar to those shown in Figure 1 were first implemented in process heaters and have been successfully used in heater applications for more than a decade. However, several factors must be considered when applying the ultra low NOx burner design to a thermal oxidiser.
Certain waste gases may include components, such as ammonia or amines, which contain chemically bound nitrogen. When bound nitrogen is burned in an excess air environment, a substantial fraction of the nitrogen is converted to NOx through a complex chain reaction. Since this reaction does not contain the high activation energy of the thermal NOx reaction, it can occur at lower temperatures. In these circumstances, the ultra low NOx burner is not as effective as a solution to mitigate the conversion of bound nitrogen to NOx. As an alternative, wastes or fuels with significant bound nitrogen are usually incinerated using a multi stage process, where an initial substoichiometric combustion zone is followed by an oxidation zone.
下载技术文件